Questions? Comments? Please contact Dr. Phillip McClean or Christina Johnson.
|
| OVERVIEW |
| Flythrough Tour |
| ~~~~~~~~~~~~ |
| MOLECULAR PROCESSES |
| Transcription |
| Regulated Transcription |
| mRNA Processing |
| mRNA Splicing |
| Translation |
| Lac Operon |
| ~~~~~~~~~~~~ |
| CELLULAR PROCESSES |
| Protein Trafficking |
| Protein Modification |
| Protein Recycling |
| Insulin Signaling |
| Constitutive Secretion |
| Regulated Secretion |
| Mitochondrial Protein Transport |
| Mitosis |
| Meiosis |
| ~~~~~~~~~~~~ |
| CELLULAR ENERGY CONVERSION |
| Atp Synthase (Gradients) |
| Electron Transport Chain |
| Photosynthesis (Light Reaction) |
| Photosystem II |
| Glycolysis (Overview) |
| Glycolysis (Reactions) |
| Citric Acid Cycle (Overview) |
| Citric Acid Cycle (Reactions) |
| Energy Consumption |
| ~~~~~~~~~~~~ |
| ~~~~~~~~~~~~~ |
| NDSU Virtual Cell YouTube |
| MCBE Home |
| Virtual Cell |
| WWWIC Home |
| Funding & Credits |
| ~~~~~~~~~~~~~ |
| HOME > PROTEIN MODIFICATION > FIRST LOOK |
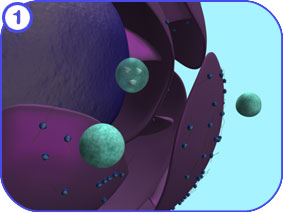
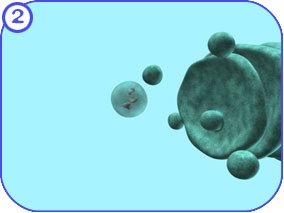
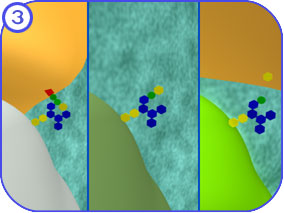
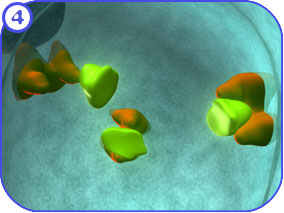
Protein Modification (Golgi): First Look The following images attempt to illustrate the major complexes and events involved in the modification of proteins that travel through the Golgi apparatus and on to the endosome. Clicking on each of the thumbnail images will bring up a larger, labeled version of the described scene. To see the Flash movie for the following sequence of images, click here. |

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.